A stunning display of natural beauty, peacock ore has a prized place in many collections. It’s iridescent and mysterious, and always found in a somewhat rough form which makes it a prized specimen.
But what is it, where does it come from, and why does it display such bold colors?
Keep reading, we’re going to dig into the matter and help you understand with our guide to peacock ore.
What is Peacock Ore?
Peacock Ore is kind of a misnomer, we actually have two different minerals that end up with the same effect. These are bornite and chalcopyrite.
These minerals often occur within the same deposits, further confusing the matter. Bornite is an important copper ore, which is why the surface bears such incredible coloration. It’s simply uneven oxidation of copper.
For a demonstration at home, grab a piece of scrap copper and hit it with a torch. You’ll quickly see that many colors come along as the heat changes, with natural bornite this uneven oxidation has occurred over a long time and it comes out of the ground that way.
Not all bornite will be peacock ore coming out of the ground, but specimens with great, natural coloration are often found that way.
Keep in mind: most peacock ore is sold as bornite… but is in reality chalcopyrite.

On the other hand, chalcopyrite resembles iron pyrite. It has a tetragonal crystalline structure, a brass coloration, and occurs in large-grained masses as well as smaller crystals. Chalcopyrite isn’t quite as easily oxidized as bornite.
Some specimens do bear color, but the strong colors associated with peacock ore are rarely found on the mineral as-is.
Instead, simple acid treatment can cause chalcopyrite to gain strong iridescent colors. It’s actually an easy process that I’ll cover before we’re done, just in case you want to touch up the colors on your own samples.
In both cases, the stone cannot be tumbled, cut, or otherwise altered without losing the coloration present on the surface of the stone. This oxidized layer is only a few micrograms thick, and it’ll come off with any sort of abrasive action.
This is compounded by their softness. Bornite is at 3 and chalcopyrite at 3.5 on the Moh’s scale. You can literally work them with a normal steel file if you had the patience, but it’ll still rip the coloration off the surface of the stone.
You can use this to your advantage if you want to know which mineral you have in front of you. Scrape a piece off with a file, or take a bit off with a chisel, and set it out for a few days. Bornite will oxidize during that time period, chalcopyrite will retain its brass color with very little difference in coloration.
Where is Peacock Ore Found Found?

Both bornite and chalcopyrite are relatively common throughout the planet. Some places are better than others of course.
Two sites are of particular note in the United States. Butte, Montana, and Bristol, Connecticut are apparently near the best deposits but specimen-grade pieces can be found across a lot of the Southwestern states as well. Arizona seems to be home to some great specimens.
These minerals tend to occur in massive formations. If you’re looking for something closer to home, then I recommend looking for copper mines and then researching the ores found there.
You should then be able to access local gravel fields, road cutouts, and other common places to find stones. Most currently functioning copper mines are going to be off-limits, and abandoned mines are dangerous places for amateurs.
Bornite will often bear some color in the field, most chalcopyrite will simply be the dull gold color it’s noted for. Neither is hard to identify, since both have distinctive formations and coloration that makes them immediately noticeable.
Remember that chalcopyrite has to be treated to get its coloration. Naturally colored bornite specimens are often the prize people are looking for, but you can still get a great display piece out of some chalcopyrite if you care to.
Bornite will sometimes have a brassy color when initially pulled from the ground, especially if you had to dig for it. It will develop the colorful patina within a few days of exposure to air, there’s no need to “jumpstart” the process.
Acid Treatment of Chalcopyrite
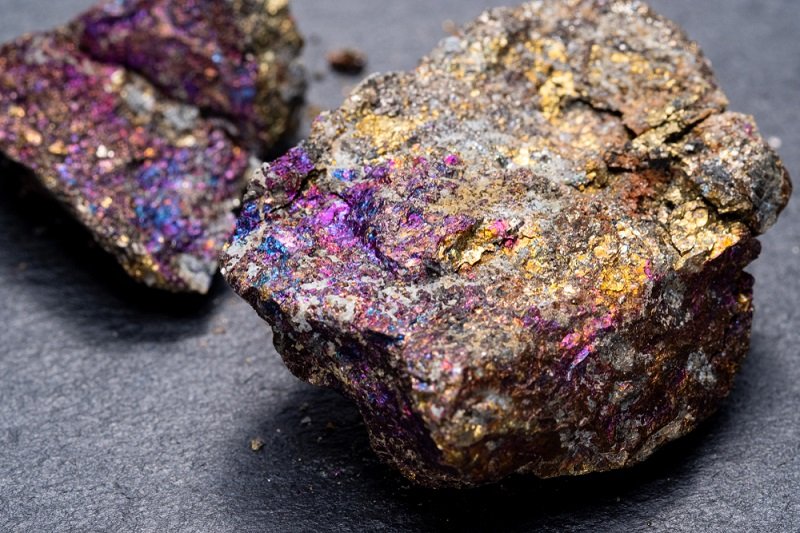
If you have a specimen of chalcopyrite that’s lost its color, you can get it back with ease. You don’t need anything special either, and it’s a safe process to do at home.
You’ll need a jar and some white vinegar. String or a bit of thin wire can help make it easier but they aren’t really necessary as long as you have a way to get the sample off the bottom of the jar.
Clean the chalcopyrite as well as possible, then fill the jar with vinegar. Drop the sample in, close the jar, and keep an eye on it. Samples will start to turn colors quickly and develop a patina over the course of a few hours.
You can speed this up by heating it on the stove but be careful of the fumes. They don’t just smell bad when you’ve got the heat up, they can also be a lung irritant. Use your stove’s hood and keep the pot capped to avoid any problems.
On occasion, a specimen will lose its color just from being handled too much. The oxidation layer is only a few atoms thick after all. In that case, you can return the mineral to its oxidized state by going through this process.
Please disclose the treatment if you’re selling to others. Most serious rockhounds already know that most “peacock ore” is treated chalcopyrite but newbies are often disappointed to find out that the stone was treated.
Still, it’s not much of a modification and they make incredible display pieces on occasion.
And hey, now you also know how to maintain good coloration if your chalcopyrite starts to fade!
- Online rock and mineral club for collectors of all levels!
- Find community with like-minded rock and mineral enthusiasts.
- Monthly Giveaways!
- Free Access to Entire Digital Library of Products (annual memberships)


